FOR UK HEALTHCARE PROFESSIONALS ONLY
Please click here for Prescribing Information and further information about Cetraxal® (ciprofloxacin) and Cetraxal Plus® (ciprofloxacin/fluocinolone).
Adverse event reporting can be found at the bottom of the page.
About AOE
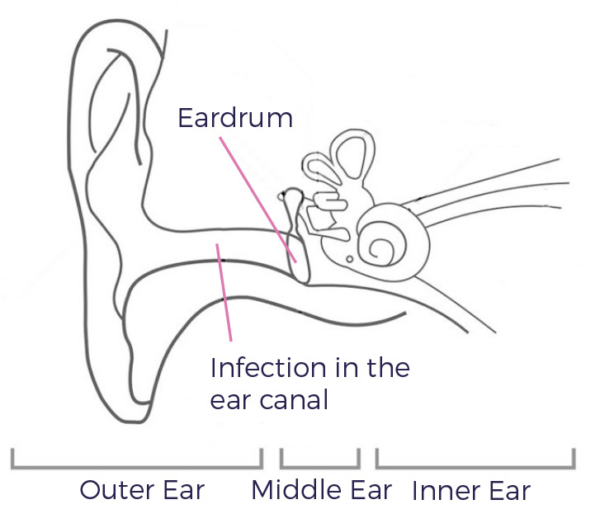
Acute Otitis Externa (AOE)
Definition: An inflammation of the external ear canal that has lasted for less than 6 weeks.1
Symptoms1:
- Otalgia
- Itching
- Fullness
- Hearing loss
- Otorrhoea
- Scaly skin in and around the ear canal
AOE
- Affects up to 10% of the population at some point in their lives2
- Is mostly caused by bacterial infection, with Pseudomonas aeruginosa and Staphylococcus aureus being most commonly isolated3
- Is also known as ‘swimmer’s ear’
- Frequent exposure to water can alter the pH of the cerumen, increasing the likelihood of bacterial infections2.
The Cetraxal range provides a non-ototoxic treatment option and may offer an alternative where antibiotic resistance is a complication
Ototoxicity
Definition: A pharmacological adverse reaction affecting the inner ear or auditory nerve, characterized by cochlear or vestibular dysfunction4.
Aminoglycosides are well known to cause ototoxicity5:
- Gentamicin: vestibulotoxic6
- Can result in dizziness, ataxia and nystagmus
- Neomycin: cochleotoxic6
- Can lead to permanent hearing loss
Fluoroquinolones eg. Cetraxal range are considered to be non-ototoxic7
Fight the fight against AOE
References
- Nice clinical Knowledge summary: Otitis Externa (Accessed April 2025)
- Rosenfeld M et al. Clinical practice guideline: acute otitis externa Otolaryngology Head Neck Surg 2014.
- Roland P. et al Microbiology of acute otitis externa. Laryngoscope. 2002;112(7):1166–77.
- Ganesan, P. et al. (2018) Ototoxicity: A Challenge in Diagnosis and Treatment. JAudiol Otol. 2018 Apr; 22(2): 59–68.
- Bitner-Glindzicz, M. et al. Ototoxicity caused by aminoglycosides. BMJ. 2007 Oct 20; 335(7624): 784–785.
- Huth, M.E. et al. (2011) Mechanisms of Aminoglycoside Ototoxicity and Targets of Hair Cell Protection. Int J Otolaryngol. 2011: 937861.
- Coates, H. Ear drops and ototoxicity. Aust Prescr 2008; 31:40-1.
Adverse events should be reported. Reporting forms and information can be found at:
www.mhra.gov.uk/yellowcard
Adverse events should also be reported to Aspire Pharma Ltd on 01730 231148.
CTX1010385C3_JUN2025