FOR UK HEALTHCARE PROFESSIONALS ONLY
Please click here for Prescribing Information and further information about Cetraxal® (ciprofloxacin) and Cetraxal Plus® (ciprofloxacin/fluocinolone).
Adverse event reporting can be found at the bottom of the page.
Clinical Data
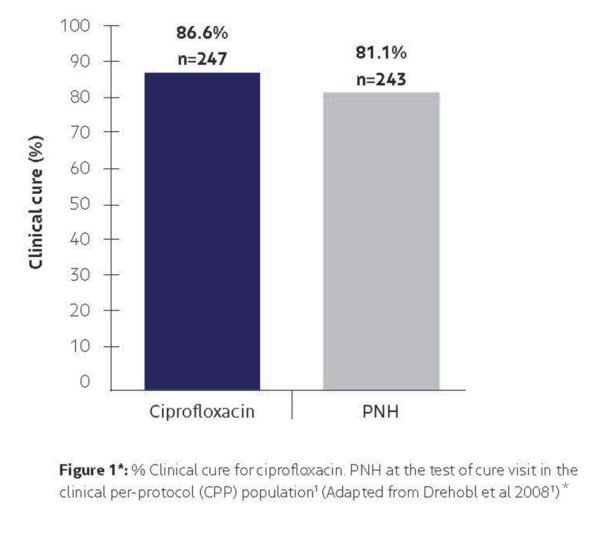
Drehobl et al (2008)1
Non-inferiority study comparing the efficacy and safety of ciprofloxacin otic solution 0.2% to polymyxin B-neomycin-hydrocortisone (PNH) otic solution in the treatment of acute diffuse otitis externa in children, adolescents and adults. 630 patients were randomised to 7 days treatment with either Cetraxal solution 0.25ml twice-daily or PNH three times daily (n=630).
✓ Ciprofloxacin was found to be non-inferior to PNH (polymyxin-b-neomycin-hydrocortisone) in achieving clinical cure. Clinical cure was defined as absence of ear pain, swelling, and discharge in patients who previously had these symptoms moderately to severely.
✓ Ciprofloxacin was well tolerated with only mild adverse events recorded: ear pruritus (0.9%), headache (0.6%) and application site pain (0.6%).
Cetraxal showed non-inferior efficacy to neomycin-based treatment, PNH
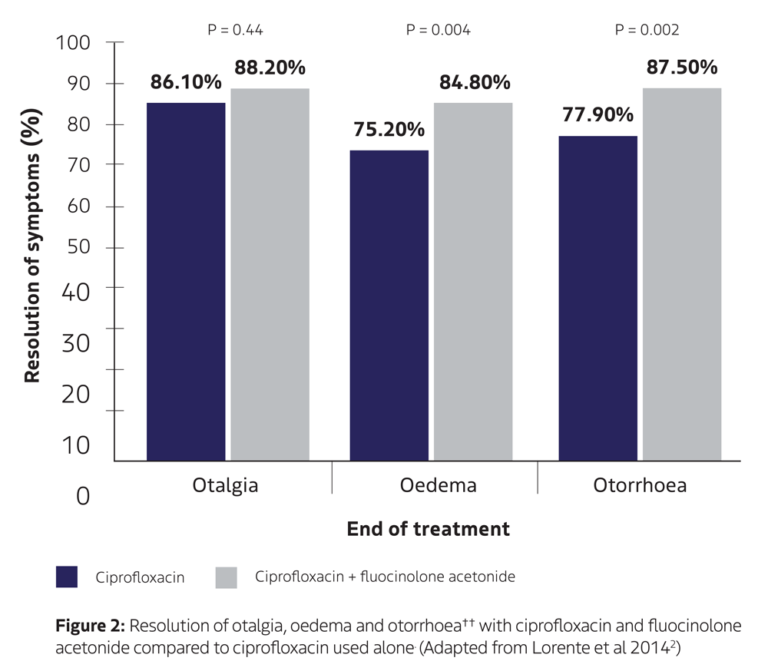
Lorente et al (2014)2
Randomised, double-blind, parallel-group clinical trial comparing the efficacy of ciprofloxacin otic solution 0.3% (4-6 drops every 8 hours for 8 days) with ciprofloxacin 0.3% and fluocinolone acetonide 0.025% otic solution (4-6 drops every 8 hours for 8 days)† in the treatment of diffuse otitis externa. Trial included children aged 7 plus, adolescents, and adults (n=590).
✓ Significantly greater reduction in intensity of oedema and otorrhoea in patients receiving ciprofloxacin + fluocinolone vs ciprofloxacin alone. ††
✓ Comparable resolution of otalgia between ciprofloxacin and ciprofloxacin + fluocinolone.
✓ Both treatments were well tolerated. Common adverse events include ear pain, ear discomfort, ear pruritus, dysguesia.
Use of steroid may be reserved for more complicated cases of AOE with swelling or known tympanic membrane perforation.
When AOE strikes, deploy Cetraxal
*Clinical cure was defined as absence of ear pain, swelling and discharge in patients who previously had these symptoms moderately or severely.
† Cetraxal Plus is licensed for use as one ampoule twice daily for seven days
† † Reduction of otalgia, oedema and otorrhoea based on visual analogue scale (VAS) score ranging from 0-10. A VAS score of 7-10 was classed as severe (or 3), 3.5-7 was classed as moderate (or 2), 0-3.5 was classed as mild (or 1) and 0 was classed as absent (or 0). The total symptom score was the sum of these three scores, ranging from 0-9
References
- Drehobl, M. et al. (2008) Comparison of efficacy and safety of ciprofloxacin otic solution 0.2% versus polymyxin B-neomycin-hydrocortisone in the treatment of acute diffuse otitis externa.Current Medical 24,(12): pp. 3531-3542.
- Lorente, J et. al; Ciprofloxacin plus fluocinolone acetonide versus ciprofloxacin alone in the treatment of diffuse otitis externa. The Journal of Laryngology & Otology (2014), 128, 591-598.
Adverse events should be reported. Reporting forms and information can be found at:
www.mhra.gov.uk/yellowcard
Adverse events should also be reported to Aspire Pharma Ltd on 01730 231148.
CTX1010385D3_JUN2025