FOR UK HEALTHCARE PROFESSIONALS ONLY
Please click here for Prescribing Information and further information about Cetraxal® (ciprofloxacin) and Cetraxal Plus® (ciprofloxacin/fluocinolone).
Adverse event reporting can be found at the bottom of the page.
Materials
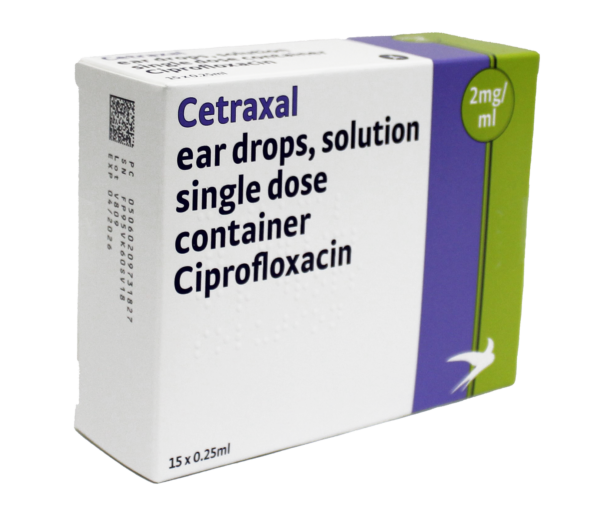
Legal category: POM
Active substance: ciprofloxacin (2mg/ml)
By using the link below you will leave the Cetraxal website and be re-directed to an external site; Aspire Pharma is not responsible for the content on external websites.
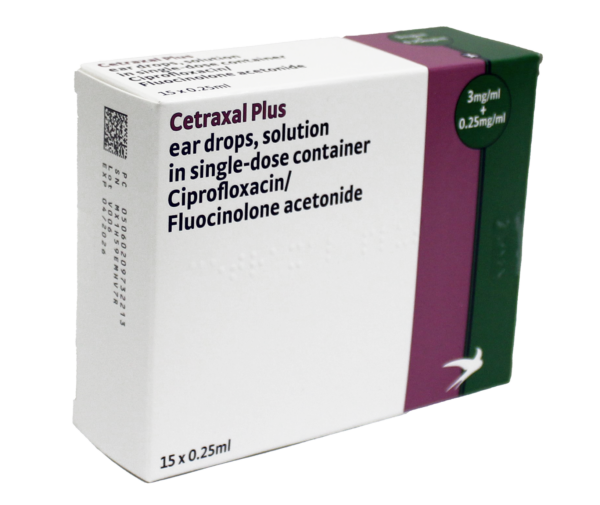
Legal category: POM
Active substance: ciprofloxacin + fluocinolone acetonide (3mg/ml + 0.25mg/ml)
By using the link below you will leave the Cetraxal website and be re-directed to an external site; Aspire Pharma is not responsible for the content on external websites.
Downloads
Range Overview
A detailed overview of the Cetraxal range.
Please email [email protected] to order copies.
Patient Guide
A guide for patients on AOE and treatment with Cetraxal and Cetraxal Plus.
Please email [email protected] to order copies.
Targeted, effective treatment for AOE
Adverse events should be reported. Reporting forms and information can be found at:
www.mhra.gov.uk/yellowcard
Adverse events should also be reported to Aspire Pharma Ltd on 01730 231148.
CTX1010385G4_JUN2025