FOR UK HEALTHCARE PROFESSIONALS ONLY
Please click here for Prescribing Information and further information about Cetraxal® (ciprofloxacin) and Cetraxal Plus® (ciprofloxacin/fluocinolone).
Adverse event reporting can be found at the bottom of the page.
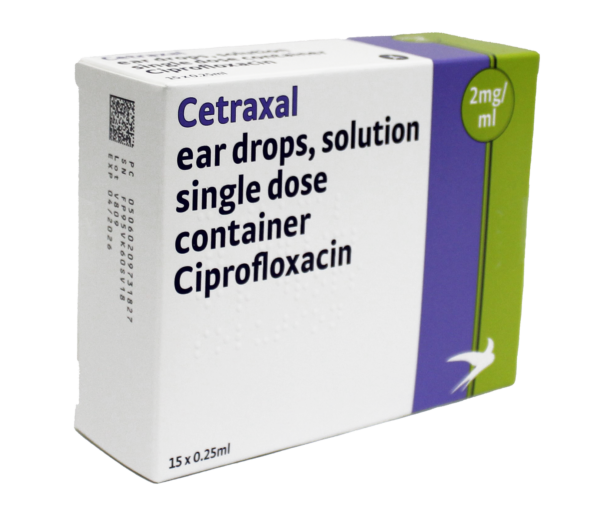
About Cetraxal
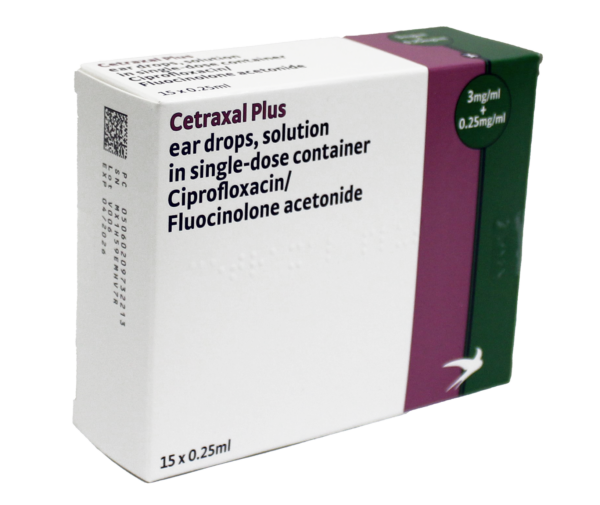
The Cetraxal range is considered to be non-ototoxic1 with Cetraxal Plus suitable for use where there is swelling or known or suspected tympanic membrane perforation.
Find out more about the Cetraxal range’s non-ototoxic properties here
Single-dose ampoules enable:
✓ Accurate dosing4
✓ Low risk of cross contamination4
✓ Easy, targeted administration: a study found 94.4% of patients found it easy to administer4
✓ Support of treatment adherence : simple, one ampoule per ear bi-daily dosing regimen
Free from preservatives and with no known sensitisers, the Cetraxal range is well tolerated whilst still being efficacious. Most common adverse events: ear pain, ear discomfort, ear pruritus, dysgeusia.2,3
Treatment options can be tailored to the patient. Cetraxal offers a steroid-free option and Cetraxal Plus is suitable for use in AOE where tympanic membrane perforation is present or suspected.2,3
NICE advises consideration of the following factors when prescribing a topical antibiotic 5:
| Status of eardrum (perforated or intact) | ✓ Cetraxal Plus can be used with a perforated tympanic membrane |
| Personal preference | ✓ The Cetraxal range allows for a tailored treatment choice |
| Cost | ✓ £6.01 per treatment course6 |
| Dosing | ✓ The Cetraxal range uses single dose ampoules for bi-daily dosing |
| Risk of adverse events: – Fungal super-infection – Aminoglycoside-induced: – Ototoxicity – Skin sensitization |
✓ The Cetraxal range is non-ototoxic and preservative-free with no known sensitising agents |
The Cetraxal Range
For uncomplicated AOE with:
For more symptomatic AOE with:
Ototoxicity
Definition: A pharmacological adverse reaction affecting the inner ear or auditory nerve, characterized by cochlear or vestibular dysfunction.7
Aminoglycosides are well known to cause ototoxicity8:
- Gentamicin: vestibulotoxic9
- Can result in dizziness, ataxia and nystagmus
- Neomycin: cochleotoxic9
- Can lead to permanent hearing loss
The Cetraxal range provides a non-ototoxic treatment option and may offer an alternative where antimicrobial resistance (AMR) is a complication.
Fluoroquinolones eg. Cetraxal range are considered to be non-ototoxic1
Antimicrobial resistance
- In a study of 144 swabs taken from patients with AOE the most commonly identified pathogen was Pseudomonas aeruginosa (45.1%)10:
- All P. aeruginosa isolates were resistant to neomycin
- All P. aeruginosa isolates were sensitive to ciprofloxacin eg. Cetraxal range
Fighting AOE, one dose at a time.
References
- Coates, H. Ear drops and ototoxicity. Aust Prescr 2008; 31:40-1.
- Cetraxal Plus SmPC: https://www.medicines.org.uk/emc/product/8847/smpc#gref [Accessed April 2025]
- Cetraxal Plus SmPC: https://www.medicines.org.uk/emc/product/9805/smpc. [Accessed April 2025]
- Drehobl, M. et al. (2008) Comparison of efficacy and safety of ciprofloxacin otic solution 0.2% versus polymyxin B-neomycin-hydrocortisone in the treatment of acute diffuse otitis externa. Current Medical 24,(12): pp. 3531-3542.
- NICE. Clinical Knowledge Summary: Otitis Externa. Accessed April 2025. Available at: https://cks.nice.org.uk/topics/otitis-externa/
- Mims.co.uk (accessed April 2025)
- Ganesan, P. et al. (2018) Ototoxicity: A Challenge in Diagnosis and Treatment. JAudiol Otol. 2018 Apr; 22(2): 59–685.
- Bitner-Glindzicz, M. et al. Ototoxicity caused by aminoglycosides. BMJ. 2007 Oct 20; 335(7624): 784–785.
- Huth, M.E. et al. (2011) Mechanisms of Aminoglycoside Ototoxicity and Targets of Hair Cell Protection. Int J Otolaryngol. 2011: 937861.
- Ninkovic, G. et al, Microbiology of otitis externa in the secondary care in United Kingdom and antimicrobial sensitivity, Auris Nasus Larynx 35 (2008) 480
Adverse events should be reported. Reporting forms and information can be found at:
www.mhra.gov.uk/yellowcard
Adverse events should also be reported to Aspire Pharma Ltd on 01730 231148.
CTX1010385B3_JUN2025